BIOSYNEX COVID-19 RAPID TEST-Casette Test
Dökümanı İndirA rapid test for the qualitative detection of antibodies (IgG and IgM) to SARS-CoV-2 in whole blood, serum,
or plasma.
Simple
Can be performed using whole blood (from venipuncture or fingerstick), serum, or plasma.
Fast
Results in 10 minutes
Complete kit for carrying out 25 tests
- 25 test cassettes
- 1 buffer vial
- 25 droppers
- Package insert
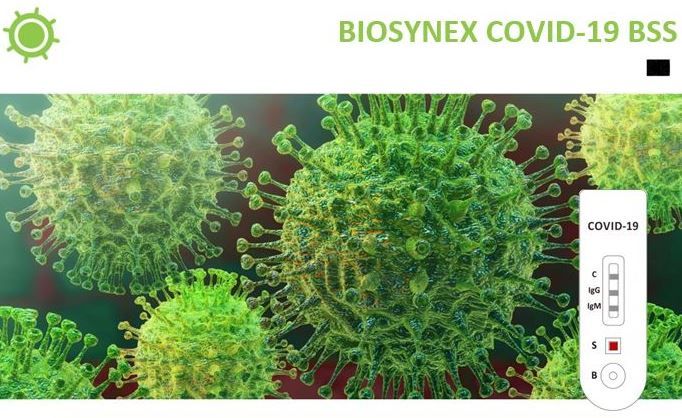
BIOSYNEX COVID-19 BSS
COVID-19 is a disease caused by a novel coronavirus (CoV) SARS-CoV-2 also known as 2019-nCoV.
The most common symptoms of COVID-19 are fever, tiredness, and dry cough. Some patients may have aches and pains, nasal congestion, runny nose, sore throat or diarrhea. These symptoms are usually mild and begin gradually.Some people become infected but don’t develop any symptoms and don't feel unwell.
Most people (about 80%) recover from the disease without needing special treatment. Around 1 out of every 6 people who gets COVID-19 becomes seriously ill and develops difficulty breathing. Most estimates of the incubation period for COVID-19 range from 1-14 days.
The BIOSYNEX COVID-19 BSS (Whole Blood/Serum/Plasma) is a rapid test that utilizes a combination of SARSCOV-2 antigen coated colored particles for the detection of IgG and IgM antibodies to SARS-COV-2 in human whole blood, serum, or plasma.
Why use a rapid test for COVID-19?
The antibody-based tests can identify people who were not known to be infected either because they never developed symptoms, or they had symptoms that were never correctly diagnosed. That means the test can identify silent infections, as well as people who were once sick but have recovered. If it turns out that many people got infected with the novel coronavirus but didn't get sick, that means the virus is less likely to be fatal than it now appears.
THE PROCEDURE

/*/*/
MATERIALS INCLUDED
BIOSYNEX COVID-19 BSS
Ref. SW40005
Contains:
- 25 test cassettes
- 1 buffer vial
- 25 droppers
- Package insert

BIBLIOGRAPHY
1. World Health Organization (WHO). WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China. Beijing: WHO;9 Jan 2020.
2. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011;81:85-164.
3. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17:181-192.
4. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. TrendsMicrobiol 2016;24:490-502.
5. Daily risk assessment on CoVid 19. European Centre for Disease Prevention and Control March 8th 2020